15 min read • Healthcare & life sciences
COVID-19 – FOCUSING ATTENTION ON THE NEXT TRANSFORMATION IN HEALTHCARE
The lightning-fast global spread of the Omicron variant of COVID-19 was a painful reminder that the only way to protect against the spread of the disease is vaccination on a global level. At the same time, South African researchers’ quick identification and analysis of Omicron demonstrated the strength and interconnectedness of the international science community, with critical data rapidly passed to those who were best able to mount a response.

Essentially the pandemic has shown the strengths and weaknesses of the global healthcare sector – and the opportunities that exist for improvement and transformation. While demonstrating what can be achieved through global collaboration, COVID-19 has also shone a harsh light on the disparities in healthcare access between developed and developing countries. To date, Africa, a continent with 1.4 billion people, has had to import 99 percent of its vaccines, resulting in low vaccination rates among its population (currently below 20 percent, or even 10 percent, in many countries1) and putting additional stress on an already-stretched healthcare system struggling with HIV and malaria.
The lack of access to innovative medicines goes far beyond vaccines, for example, to biologics such as insulin. While the discovery of insulin was made 100 years ago, access to this drug for the treatment of diabetes is still limited in many parts of the world – often where the disease is now becoming most prevalent. Only one in 10 patients in low- and middle-income countries receive comprehensive diabetic care, and even in the US, 30 percent of patients with type 1 or 2 diabetes engage in “cost-related insulin underuse”.
At the same time, governments across the world, including low- and middle-income countries, are increasingly held responsible for their populations’ health and access to relevant medicines. This pressure has been further increased by the pandemic and its focus on the performance of different healthcare systems.
These needs are driving a requirement to expand capabilities around innovation and access in low- and middle-income countries, which potentially will have transformative impact on the wider global healthcare ecosystem, including pharmaceutical companies, governments and other stakeholders. The wider industry should therefore look to understand and answer these five key questions:
-
How can low- to middle-income countries improve access to innovative medicines for their citizens?
-
What capabilities are required to rebalance the current supply chain and bring production to these countries?
-
How would an increasingly local production of vaccines and biologics change the global supply chain?
-
Are there faster and easier ways to develop, produce and distribute not only vaccines, but also other innovative drugs, in and for developing markets?
-
What new and innovative approaches are already in place, and how will this change the market, in both the developing and developed world?
COVID-19 - FOCUSING ATTENTION ON THE NEXT TRANSFORMATION IN LOCAL AND GLOBAL HEALTHCARE
The pandemic has highlighted what the global life sciences industry can achieve in a remarkably short time, with the development of a multitude of novel (and successful) vaccine approaches that were quickly rolled out around the globe.
On the negative side, the question is why it took a pandemic to enable all of those technologies at a large scale. Additionally, we have seen huge disparities in vaccine distribution. Access to vaccines and the materials required for their production and distribution have become political issues. Suddenly, governments are being judged by their citizens on their abilities to produce and/or procure and distribute vaccines, while providing access to particular vaccines has become a tool of foreign policy for countries as diverse as China, Egypt, India and Russia.
Moving beyond COVID-19, bringing innovative medicines to developing markets, and using the lessons learned to improve healthcare outcomes in the rest of the world, targets two key areas for innovation – access and manufacturing – while also considering R&D and enabling a different kind of innovation.
1. INCREASING ACCESS FOR WIDER POPULATIONS
Access to medication, whether produced locally or internationally, is a key challenge, based on factors such as cost, availability, healthcare systems, reimbursement models, and the potential difficulties of physical distribution, especially when this involves cold-chain logistics and delivery in sparsely populated areas.
The required ecosystem thinking to establish even clinical trials in these regions has recently been demonstrated through the first malaria vaccine, which was approved and recommended by the WHO. (See the box-out below.)
MALARIA
Malaria is a typical example of a disease that not only is very complex, but also mainly affects low-income and developing countries. According to the Malaria Alliance, 250,000 children die each year of the disease. Development and testing of vaccines in this space are usually supported by donations, alongside commitments by pharma companies and organizations such as the WHO and Gavi.
A new vaccine, RTS,S, developed by GSK, has recently been recommended by the WHO for use in children, following a pilot study that showed significant reduction (30 percent) in deadly severe malaria, even when introduced in areas where insecticide-treated nets are widely used and there is good access to diagnosis and treatment.
For this vaccine, the pilot study required the joint efforts of local health ministries and the WHO; financial support through funds such as Gavi, the Global Fund, and Unitaid; and donations of the vaccine itself by GSK, which invested USD 700 million in its development. Following the WHO’s recommendation, global stakeholders, including Gavi, are now considering whether and how to finance a new malaria vaccination program for countries in sub-Saharan Africa. Ahead of this decision, an innovative financing agreement has been reached between Gavi, MedAccess and GSK to guarantee continued production of the RTS,S antigen, which will be essential to ensure ongoing vaccine supply.
However, this access challenge is also driving very different innovation – for example, Zipline, a US-based drone company, recently completed the first long-range drone delivery of authorized mRNA COVID-19 vaccines requiring an ultra-cold chain in Ghana. The collaboration will allow the distribution of approximately 50,000 doses of the Pfizer-BioNTech COVID-19 vaccine in the country, pioneering a new distribution model that overcomes the twin issues of limited infrastructure and widely spread populations. This approach could ultimately be rolled out in Europe, Asia-Pacific and the Americas, especially in areas of low population density.
Availability, particularly of vaccines, has become a key consideration for national security, with the allied consideration of using healthcare as a tool for managing foreign policy. The former relates to ensuring that key medicines are available on call for all, and the latter to the possibility of substituting capital with healthcare.
Illustrating this second point, richer Middle Eastern countries – especially those within the Gulf Cooperation Council (GCC) – are large aid donors to many countries around the world, especially in Africa. They are already developing advanced healthcare infrastructure within the region to stem the loss of income if local patients fly elsewhere for treatment. This infrastructure can be used effectively as a tool of foreign policy, supplementing financial aid by providing treatment to patients from recipient countries. This emerging approach has distinct advantages for both recipients and donors. It ensures that aid reaches the person it is intended for, the efficiency of the facility increases, the case mix becomes wider and better, the possibility of diverse clinical trials increases, and it removes any inefficiencies that might result from a simple capital or goods donation.
2. BRIDGING THE PRODUCTION GAP BY THINKING LOCALLY AND GLOBALLY
The pandemic has accelerated the push for local, high-quality production of patented medications, supported by their originators, alongside open business and operating models that include partnerships with international organizations.
Production capabilities and ecosystems clearly vary widely – India is increasingly becoming the “pharmacy of the world”, introducing innovative new approaches. Yet there are currently fewer than 10 vaccine manufacturers in Africa, most of which are in Egypt, Morocco, Tunisia, Senegal, and South Africa. Steps are being taken to expand this capacity. For example, BioNTech has recently assumed a very active role in supporting Rwanda and Senegal in setting up a “vaccine hub” in the region specifically to produce malaria and tuberculosis vaccines.
Shifting the focus to South-East Asia, countries such as Thailand have long realized that their healthcare systems are being put under significant stress by:
-
High pharmaceutical spending due to a preference for imported patented drugs and branded generic drugs
-
Reliance on imported active pharmaceutical ingredients (APIs) (making up 90 percent of local API consumption) and drugs (64 percent of local drug consumption) due to the lack of local development and manufacturing capabilities and capacity
-
A lack of a pharmaceutical ecosystem in the region, with gaps in the value chain, specifically in research and development
SOLVING THE MEDICINE BOTTLENECK IN SOUTH-EAST ASIA
Thailand has identified local production as an area of both potential economic growth and a lever to reduce healthcare costs. It has made the manufacture of APIs and biosimilars part of a government initiative, working with major government-owned industry players in adjacent sectors (such as chemicals) to enable local capacity and capability for drug manufacture, which also creates new revenue streams.
This is being driven by the creation of a Thai contract development and manufacturing company (CDMO), which addresses three important areas:
1. Drug development capabilities
-
Process and scale up development, APIs, and drug formulation
-
Capability to develop large-molecule drugs (biologics)
2. Centralization of manufacturing to achieve required scale
- Production consolidation at a single manufacturing facility to distribute overhead costs and produce in a more cost-effective manner
3. Leading manufacturing capabilities and technologies
-
State-of-the-art API and drug manufacturing technologies, compliant with key regulations
-
Bioreactors to manufacture large-molecule drugs
This Thai CDMO aims to accelerate the creation of a local pharma ecosystem and enable local companies without competing with them, which benefits researchers, the wider sector, healthcare providers, and ultimately patients.
In South-East Asia, these factors are exacerbated by the changing dynamics of a market that is rapidly growing economically, driving significant growth and change in the population. This has led to various chronic health issues that are now aggravated by the uncertainty caused by the current pandemic.
While there is currently an intense discussion on releasing patents for vaccines, it is important to look at the broader question of what it will take to enable the local production and supply of innovative medicines. Beyond IP, countries looking to build local production (and local R&D) need to develop multiple capabilities alongside the investment and time required to build production facilities:
-
Universities with the freedom and resources to carry out research
-
Trained people who are able to master complex production technologies
-
Infrastructure that ensures a steady and uninterrupted supply of water and energy to run production facilities
-
Sterile production processes with extremely high-quality standards and, in many cases, a cold chain
-
Legal and regulatory frameworks to ensure that quality and liability are covered adequately
3. THE OPPORTUNITY FOR INNOVATIVE MANUFACTURING AND R&D APPROACHES TO IMPROVE ACCESS FOR ALL
When it comes to the development of home-grown pharmaceutical industries, China and India have both been through a remarkable journey in recent years. To build its capabilities, China has combined a strong push across multiple dimensions, including education in both data science and life sciences, which has created industrial infrastructure and improved regulatory frameworks. For example, the Chinese FDA used to be known for extremely slow processes in which it took several years to set up a clinical trial, but it has completely turned that around. In parallel, Chinese companies such as WuXi (a biologics contract manufacturer) and BGI Genomics (a large biotech player that was instrumental in the early sequencing of SARS-CoV-2), as well as data giant Tencent, enabled an ecosystem that allowed China to be among the first countries to develop a vaccine that could then be used both at home and as an instrument of foreign policy.
India has long been recognized as a hub for production of cheap APIs and generics, with companies such as Dr. Reddy and Ranbaxy initially leading the way. However, this should not mask the amount of innovation happening in the country, addressing areas of technological advancement that are untapped in mature and high- income markets such as Europe and North America.
With its focus on generics, vaccines (India supplies about 50 percent of vaccines globally), bio-similars, and biologics, the Indian pharma sector has been much more focused on delivering innovation that underpins robust and efficient production than on speed to market. More efficient processes mean lower prices, which in turn makes these drugs and vaccines accessible to broader populations across the world (as highlighted in the box-out below).
BIOLOGICAL E – AN INDIAN INNOVATOR
Innovation in India often focuses on drugs that are accessible from price and technological standpoints. This is demonstrated by Biological E, an innovative vaccine and pharmaceutical player that concentrates on “providing equitable access to quality vaccines and pharmaceutical products” with a clear focus on producing vaccines that are then recommended by the WHO. For the development of a COVID-19 vaccine, it looked to create a low-cost, open-source alternative to expensive and limited-supply mRNA vaccines for low- to middle-income and under-vaccinated countries. Its CORBEVAXTM vaccine was originally developed by US academics at Baylor College of Medicine and the Texas Children’s Center for Vaccine Development and provided without licensing costs/IP. The subunit vaccine only requires standard refrigeration and comes at a price as low as USD 2.50 per dose. It has received approval for emergency use in India, and Biological E is now in discussion with the WHO for recommendations. The progress of CORBEVAXTM illustrates how effective international collaboration can drive development.*
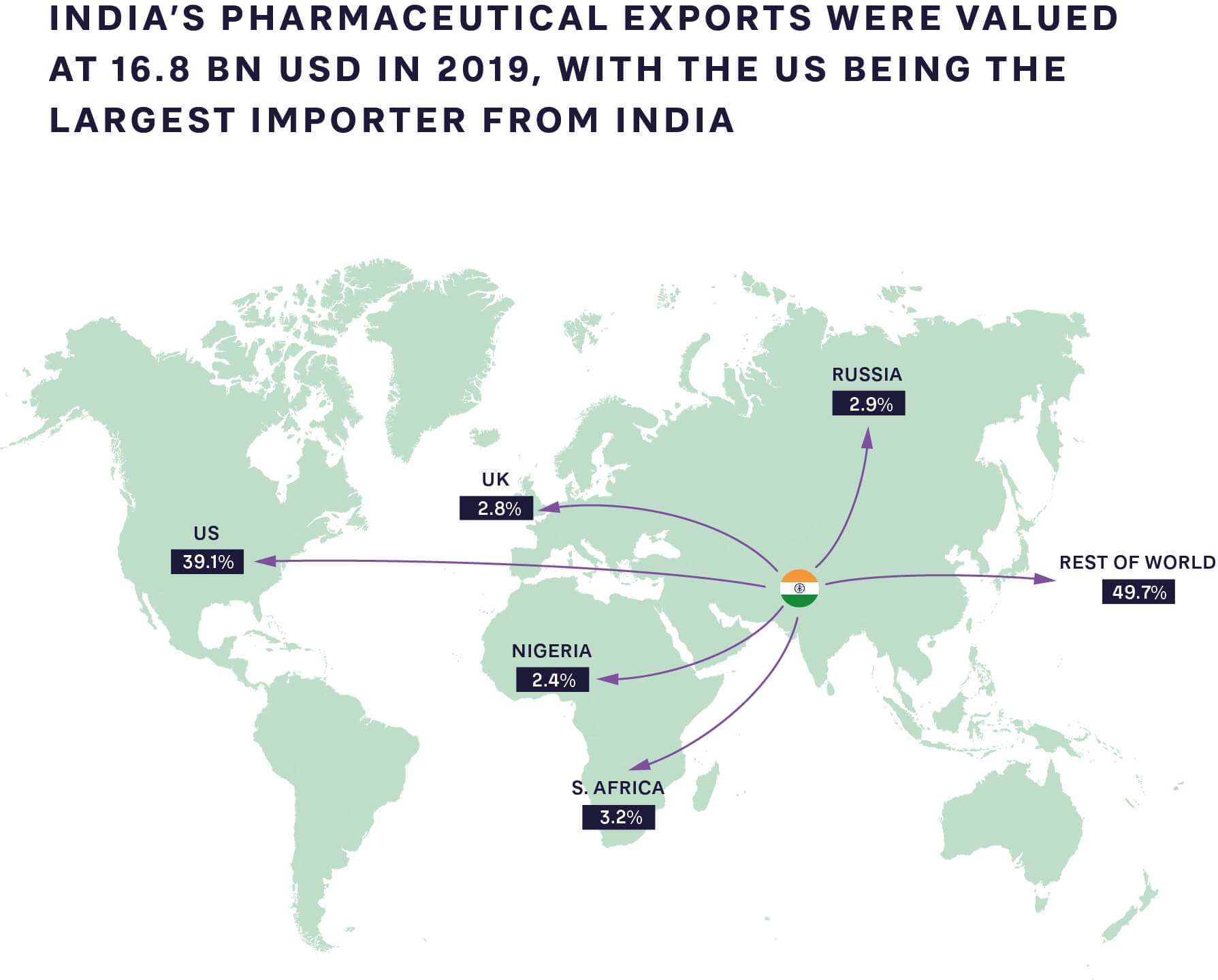
FIGURE 1: PHARMACEUTICAL EXPORTS FROM INDIA TO THE REST OF THE WORLD 2019 SOURCE: GLOBAL DATA, ARTHUR D. LITTLE ANALYSIS
Indian production innovation is also driving benefits around insulin, used for the treatment of type 1 and type 2 diabetes. The number of people with diabetes globally rose from 108 million in 1980 to 422 million in 2014, with prevalence growing more rapidly in low- and middle-income countries than in high-income ones. Overall, in 2019, diabetes was the ninth leading global cause of death, with an estimated 1.5 million deaths directly caused by the disease.
Indian company Biocon has created its “10 cents” initiative for insulin, bringing the price down from about USD 5 to just 10 cents per day for treatment in India. As part of this initiative, Biocon is the first company to develop and market insulin produced by the Pichia pastoris yeast, which allows for faster and more efficient production compared to current methods.
As can be seen from Figure 2, the cost of insulin, which is a necessity for type 1 diabetes (T1D) patients to survive and highly relevant for many type 2 diabetes patients to control blood sugar levels and avoid long-term side effects, is still high in many markets. This includes the US, which has out-of-pocket treatment costs close to USD 1,000 a month for T1D patients.
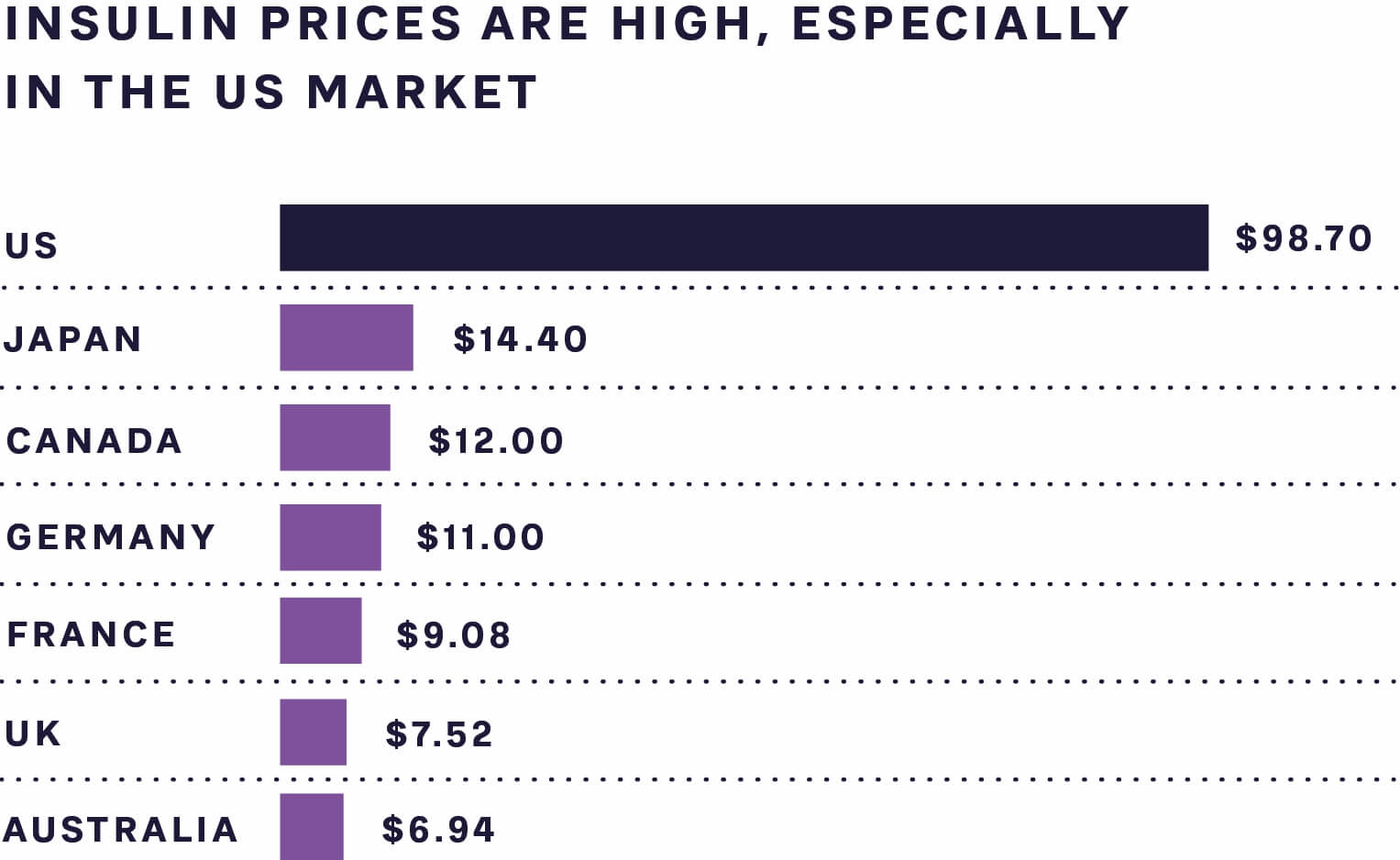
FIGURE 2: AVERAGE INSULIN PRICE PER STANDARD UNIT, 2018 SOURCE: RAND CORPORATION
Innovation is not limited to insulin - a recent Arthur D. Little study on biologics production shows that new methods have a much higher chance of adoption in countries such as India. These less “settled” markets are proving more open to innovation, as they are often still building new production systems and less bound by existing facilities and preconceptions in the market. Additionally, the fear of using a new system that might not yet have regulatory approval is much lower, meaning companies are more likely to take risks. Once proven, these approaches can then be applied within developed countries, bringing benefits in terms of decreased manufacturing costs and lower supply chain risk.
4. THE BENEFITS AND OPPORTUNITIES OF REBALANCING FOR ALL PLAYERS – THE NEXT TRANSFORMATION IN HEALTHCARE
Transforming access to innovative medicines requires a joined-up approach that shares best practice and develops new capabilities in low- and middle-income countries. As the experiences of India, China and other countries demonstrate, this rebalancing is already happening and likely to accelerate post-pandemic.
Given these trends, should established global life science companies, policymakers and governments fear this global shift led by low-cost production and innovation and new access methods across the developing world?
The answer is a resounding no. In addition to considering risk, all players should look at the value and opportunities provided to global and local healthcare by initiatives targeting, for example, the reuse of existing drugs, innovative approaches to generics and biosimilars, and even innovative distribution models such as drone delivery. Different regions bring value to different parts of the healthcare system; supporting local production and innovation for more patient-centric, but also cheaper and more accessible, medications will ultimately be a global benefit. Lessons can be learned, and innovative approaches adopted, that can be applied within the developed world. It is a two-way street – established players will be able to tap into a wider pool of talent, access new markets, lower supply chain risks and bring down manufacturing costs. This next transformation in healthcare will therefore likely be beneficial to patients globally.
INSIGHTS FOR THE EXECUTIVE
Achieving the benefits of a truly global healthcare ecosystem requires innovation from companies, policy makers and governments in three key areas:
1. Accelerate open collaboration and risk sharing across nations and companies:
- Enable open collaboration between nation states and pharmaceutical companies and other industry players to justify investments. This includes solid legal frameworks, alignment on how IP is treated and action to crack down on counterfeit/black/ grey market drugs. These frameworks should be driven and overseen by global organizations (such as the WHO) to avoid the need for individual pharma companies to align with each nation state.
- New risk-sharing models to protect companies from the inevitable challenges that will arise when building new manufacturing capability and supply chains and transferring tech and IP, while ensuring the highest quality levels are maintained. This will lower risks and costs over time as capabilities scale.
- A shift in how technologically advanced companies share knowledge and train local populations to do advanced technical work. This requires collaboration with governments, universities and NGOs to build talent pools and put safeguards in place to protect investment in training and development. Ultimately this will create a larger, more diverse global talent pool.
- Innovating and operating in new countries and markets will require governments and policymakers to invest in local education and regulations to stimulate the right ecosystem capabilities to drive local R&D, manufacturing and business model innovation. Companies, in turn, can leverage this as they seek to develop new approaches to drug development, market access and distribution in developed markets.
Ultimately, rebalancing global supply chains and bringing innovative medicines and advanced manufacturing capabilities to developing regions will transform global healthcare in five key ways:
-
Improving local and global health
-
Improving the economies of developing nations
-
Improving the trust of citizens in their governments and ability to deliver quality healthcare
-
De-risking the pharmaceutical supply chain and therefore access around the world, providing better resilience globally
-
Opening new opportunities through access to a wider, more diverse talent pool, innovative manufacturing and distribution approaches, and the ability to effectively target new markets and patient populations

15 min read • Healthcare & life sciences
COVID-19 – FOCUSING ATTENTION ON THE NEXT TRANSFORMATION IN HEALTHCARE
The lightning-fast global spread of the Omicron variant of COVID-19 was a painful reminder that the only way to protect against the spread of the disease is vaccination on a global level. At the same time, South African researchers’ quick identification and analysis of Omicron demonstrated the strength and interconnectedness of the international science community, with critical data rapidly passed to those who were best able to mount a response.

DATE

Essentially the pandemic has shown the strengths and weaknesses of the global healthcare sector – and the opportunities that exist for improvement and transformation. While demonstrating what can be achieved through global collaboration, COVID-19 has also shone a harsh light on the disparities in healthcare access between developed and developing countries. To date, Africa, a continent with 1.4 billion people, has had to import 99 percent of its vaccines, resulting in low vaccination rates among its population (currently below 20 percent, or even 10 percent, in many countries1) and putting additional stress on an already-stretched healthcare system struggling with HIV and malaria.
The lack of access to innovative medicines goes far beyond vaccines, for example, to biologics such as insulin. While the discovery of insulin was made 100 years ago, access to this drug for the treatment of diabetes is still limited in many parts of the world – often where the disease is now becoming most prevalent. Only one in 10 patients in low- and middle-income countries receive comprehensive diabetic care, and even in the US, 30 percent of patients with type 1 or 2 diabetes engage in “cost-related insulin underuse”.
At the same time, governments across the world, including low- and middle-income countries, are increasingly held responsible for their populations’ health and access to relevant medicines. This pressure has been further increased by the pandemic and its focus on the performance of different healthcare systems.
These needs are driving a requirement to expand capabilities around innovation and access in low- and middle-income countries, which potentially will have transformative impact on the wider global healthcare ecosystem, including pharmaceutical companies, governments and other stakeholders. The wider industry should therefore look to understand and answer these five key questions:
-
How can low- to middle-income countries improve access to innovative medicines for their citizens?
-
What capabilities are required to rebalance the current supply chain and bring production to these countries?
-
How would an increasingly local production of vaccines and biologics change the global supply chain?
-
Are there faster and easier ways to develop, produce and distribute not only vaccines, but also other innovative drugs, in and for developing markets?
-
What new and innovative approaches are already in place, and how will this change the market, in both the developing and developed world?
COVID-19 - FOCUSING ATTENTION ON THE NEXT TRANSFORMATION IN LOCAL AND GLOBAL HEALTHCARE
The pandemic has highlighted what the global life sciences industry can achieve in a remarkably short time, with the development of a multitude of novel (and successful) vaccine approaches that were quickly rolled out around the globe.
On the negative side, the question is why it took a pandemic to enable all of those technologies at a large scale. Additionally, we have seen huge disparities in vaccine distribution. Access to vaccines and the materials required for their production and distribution have become political issues. Suddenly, governments are being judged by their citizens on their abilities to produce and/or procure and distribute vaccines, while providing access to particular vaccines has become a tool of foreign policy for countries as diverse as China, Egypt, India and Russia.
Moving beyond COVID-19, bringing innovative medicines to developing markets, and using the lessons learned to improve healthcare outcomes in the rest of the world, targets two key areas for innovation – access and manufacturing – while also considering R&D and enabling a different kind of innovation.
1. INCREASING ACCESS FOR WIDER POPULATIONS
Access to medication, whether produced locally or internationally, is a key challenge, based on factors such as cost, availability, healthcare systems, reimbursement models, and the potential difficulties of physical distribution, especially when this involves cold-chain logistics and delivery in sparsely populated areas.
The required ecosystem thinking to establish even clinical trials in these regions has recently been demonstrated through the first malaria vaccine, which was approved and recommended by the WHO. (See the box-out below.)
MALARIA
Malaria is a typical example of a disease that not only is very complex, but also mainly affects low-income and developing countries. According to the Malaria Alliance, 250,000 children die each year of the disease. Development and testing of vaccines in this space are usually supported by donations, alongside commitments by pharma companies and organizations such as the WHO and Gavi.
A new vaccine, RTS,S, developed by GSK, has recently been recommended by the WHO for use in children, following a pilot study that showed significant reduction (30 percent) in deadly severe malaria, even when introduced in areas where insecticide-treated nets are widely used and there is good access to diagnosis and treatment.
For this vaccine, the pilot study required the joint efforts of local health ministries and the WHO; financial support through funds such as Gavi, the Global Fund, and Unitaid; and donations of the vaccine itself by GSK, which invested USD 700 million in its development. Following the WHO’s recommendation, global stakeholders, including Gavi, are now considering whether and how to finance a new malaria vaccination program for countries in sub-Saharan Africa. Ahead of this decision, an innovative financing agreement has been reached between Gavi, MedAccess and GSK to guarantee continued production of the RTS,S antigen, which will be essential to ensure ongoing vaccine supply.
However, this access challenge is also driving very different innovation – for example, Zipline, a US-based drone company, recently completed the first long-range drone delivery of authorized mRNA COVID-19 vaccines requiring an ultra-cold chain in Ghana. The collaboration will allow the distribution of approximately 50,000 doses of the Pfizer-BioNTech COVID-19 vaccine in the country, pioneering a new distribution model that overcomes the twin issues of limited infrastructure and widely spread populations. This approach could ultimately be rolled out in Europe, Asia-Pacific and the Americas, especially in areas of low population density.
Availability, particularly of vaccines, has become a key consideration for national security, with the allied consideration of using healthcare as a tool for managing foreign policy. The former relates to ensuring that key medicines are available on call for all, and the latter to the possibility of substituting capital with healthcare.
Illustrating this second point, richer Middle Eastern countries – especially those within the Gulf Cooperation Council (GCC) – are large aid donors to many countries around the world, especially in Africa. They are already developing advanced healthcare infrastructure within the region to stem the loss of income if local patients fly elsewhere for treatment. This infrastructure can be used effectively as a tool of foreign policy, supplementing financial aid by providing treatment to patients from recipient countries. This emerging approach has distinct advantages for both recipients and donors. It ensures that aid reaches the person it is intended for, the efficiency of the facility increases, the case mix becomes wider and better, the possibility of diverse clinical trials increases, and it removes any inefficiencies that might result from a simple capital or goods donation.
2. BRIDGING THE PRODUCTION GAP BY THINKING LOCALLY AND GLOBALLY
The pandemic has accelerated the push for local, high-quality production of patented medications, supported by their originators, alongside open business and operating models that include partnerships with international organizations.
Production capabilities and ecosystems clearly vary widely – India is increasingly becoming the “pharmacy of the world”, introducing innovative new approaches. Yet there are currently fewer than 10 vaccine manufacturers in Africa, most of which are in Egypt, Morocco, Tunisia, Senegal, and South Africa. Steps are being taken to expand this capacity. For example, BioNTech has recently assumed a very active role in supporting Rwanda and Senegal in setting up a “vaccine hub” in the region specifically to produce malaria and tuberculosis vaccines.
Shifting the focus to South-East Asia, countries such as Thailand have long realized that their healthcare systems are being put under significant stress by:
-
High pharmaceutical spending due to a preference for imported patented drugs and branded generic drugs
-
Reliance on imported active pharmaceutical ingredients (APIs) (making up 90 percent of local API consumption) and drugs (64 percent of local drug consumption) due to the lack of local development and manufacturing capabilities and capacity
-
A lack of a pharmaceutical ecosystem in the region, with gaps in the value chain, specifically in research and development
SOLVING THE MEDICINE BOTTLENECK IN SOUTH-EAST ASIA
Thailand has identified local production as an area of both potential economic growth and a lever to reduce healthcare costs. It has made the manufacture of APIs and biosimilars part of a government initiative, working with major government-owned industry players in adjacent sectors (such as chemicals) to enable local capacity and capability for drug manufacture, which also creates new revenue streams.
This is being driven by the creation of a Thai contract development and manufacturing company (CDMO), which addresses three important areas:
1. Drug development capabilities
-
Process and scale up development, APIs, and drug formulation
-
Capability to develop large-molecule drugs (biologics)
2. Centralization of manufacturing to achieve required scale
- Production consolidation at a single manufacturing facility to distribute overhead costs and produce in a more cost-effective manner
3. Leading manufacturing capabilities and technologies
-
State-of-the-art API and drug manufacturing technologies, compliant with key regulations
-
Bioreactors to manufacture large-molecule drugs
This Thai CDMO aims to accelerate the creation of a local pharma ecosystem and enable local companies without competing with them, which benefits researchers, the wider sector, healthcare providers, and ultimately patients.
In South-East Asia, these factors are exacerbated by the changing dynamics of a market that is rapidly growing economically, driving significant growth and change in the population. This has led to various chronic health issues that are now aggravated by the uncertainty caused by the current pandemic.
While there is currently an intense discussion on releasing patents for vaccines, it is important to look at the broader question of what it will take to enable the local production and supply of innovative medicines. Beyond IP, countries looking to build local production (and local R&D) need to develop multiple capabilities alongside the investment and time required to build production facilities:
-
Universities with the freedom and resources to carry out research
-
Trained people who are able to master complex production technologies
-
Infrastructure that ensures a steady and uninterrupted supply of water and energy to run production facilities
-
Sterile production processes with extremely high-quality standards and, in many cases, a cold chain
-
Legal and regulatory frameworks to ensure that quality and liability are covered adequately
3. THE OPPORTUNITY FOR INNOVATIVE MANUFACTURING AND R&D APPROACHES TO IMPROVE ACCESS FOR ALL
When it comes to the development of home-grown pharmaceutical industries, China and India have both been through a remarkable journey in recent years. To build its capabilities, China has combined a strong push across multiple dimensions, including education in both data science and life sciences, which has created industrial infrastructure and improved regulatory frameworks. For example, the Chinese FDA used to be known for extremely slow processes in which it took several years to set up a clinical trial, but it has completely turned that around. In parallel, Chinese companies such as WuXi (a biologics contract manufacturer) and BGI Genomics (a large biotech player that was instrumental in the early sequencing of SARS-CoV-2), as well as data giant Tencent, enabled an ecosystem that allowed China to be among the first countries to develop a vaccine that could then be used both at home and as an instrument of foreign policy.
India has long been recognized as a hub for production of cheap APIs and generics, with companies such as Dr. Reddy and Ranbaxy initially leading the way. However, this should not mask the amount of innovation happening in the country, addressing areas of technological advancement that are untapped in mature and high- income markets such as Europe and North America.
With its focus on generics, vaccines (India supplies about 50 percent of vaccines globally), bio-similars, and biologics, the Indian pharma sector has been much more focused on delivering innovation that underpins robust and efficient production than on speed to market. More efficient processes mean lower prices, which in turn makes these drugs and vaccines accessible to broader populations across the world (as highlighted in the box-out below).
BIOLOGICAL E – AN INDIAN INNOVATOR
Innovation in India often focuses on drugs that are accessible from price and technological standpoints. This is demonstrated by Biological E, an innovative vaccine and pharmaceutical player that concentrates on “providing equitable access to quality vaccines and pharmaceutical products” with a clear focus on producing vaccines that are then recommended by the WHO. For the development of a COVID-19 vaccine, it looked to create a low-cost, open-source alternative to expensive and limited-supply mRNA vaccines for low- to middle-income and under-vaccinated countries. Its CORBEVAXTM vaccine was originally developed by US academics at Baylor College of Medicine and the Texas Children’s Center for Vaccine Development and provided without licensing costs/IP. The subunit vaccine only requires standard refrigeration and comes at a price as low as USD 2.50 per dose. It has received approval for emergency use in India, and Biological E is now in discussion with the WHO for recommendations. The progress of CORBEVAXTM illustrates how effective international collaboration can drive development.*

FIGURE 1: PHARMACEUTICAL EXPORTS FROM INDIA TO THE REST OF THE WORLD 2019 SOURCE: GLOBAL DATA, ARTHUR D. LITTLE ANALYSIS
Indian production innovation is also driving benefits around insulin, used for the treatment of type 1 and type 2 diabetes. The number of people with diabetes globally rose from 108 million in 1980 to 422 million in 2014, with prevalence growing more rapidly in low- and middle-income countries than in high-income ones. Overall, in 2019, diabetes was the ninth leading global cause of death, with an estimated 1.5 million deaths directly caused by the disease.
Indian company Biocon has created its “10 cents” initiative for insulin, bringing the price down from about USD 5 to just 10 cents per day for treatment in India. As part of this initiative, Biocon is the first company to develop and market insulin produced by the Pichia pastoris yeast, which allows for faster and more efficient production compared to current methods.
As can be seen from Figure 2, the cost of insulin, which is a necessity for type 1 diabetes (T1D) patients to survive and highly relevant for many type 2 diabetes patients to control blood sugar levels and avoid long-term side effects, is still high in many markets. This includes the US, which has out-of-pocket treatment costs close to USD 1,000 a month for T1D patients.

FIGURE 2: AVERAGE INSULIN PRICE PER STANDARD UNIT, 2018 SOURCE: RAND CORPORATION
Innovation is not limited to insulin - a recent Arthur D. Little study on biologics production shows that new methods have a much higher chance of adoption in countries such as India. These less “settled” markets are proving more open to innovation, as they are often still building new production systems and less bound by existing facilities and preconceptions in the market. Additionally, the fear of using a new system that might not yet have regulatory approval is much lower, meaning companies are more likely to take risks. Once proven, these approaches can then be applied within developed countries, bringing benefits in terms of decreased manufacturing costs and lower supply chain risk.
4. THE BENEFITS AND OPPORTUNITIES OF REBALANCING FOR ALL PLAYERS – THE NEXT TRANSFORMATION IN HEALTHCARE
Transforming access to innovative medicines requires a joined-up approach that shares best practice and develops new capabilities in low- and middle-income countries. As the experiences of India, China and other countries demonstrate, this rebalancing is already happening and likely to accelerate post-pandemic.
Given these trends, should established global life science companies, policymakers and governments fear this global shift led by low-cost production and innovation and new access methods across the developing world?
The answer is a resounding no. In addition to considering risk, all players should look at the value and opportunities provided to global and local healthcare by initiatives targeting, for example, the reuse of existing drugs, innovative approaches to generics and biosimilars, and even innovative distribution models such as drone delivery. Different regions bring value to different parts of the healthcare system; supporting local production and innovation for more patient-centric, but also cheaper and more accessible, medications will ultimately be a global benefit. Lessons can be learned, and innovative approaches adopted, that can be applied within the developed world. It is a two-way street – established players will be able to tap into a wider pool of talent, access new markets, lower supply chain risks and bring down manufacturing costs. This next transformation in healthcare will therefore likely be beneficial to patients globally.
INSIGHTS FOR THE EXECUTIVE
Achieving the benefits of a truly global healthcare ecosystem requires innovation from companies, policy makers and governments in three key areas:
1. Accelerate open collaboration and risk sharing across nations and companies:
- Enable open collaboration between nation states and pharmaceutical companies and other industry players to justify investments. This includes solid legal frameworks, alignment on how IP is treated and action to crack down on counterfeit/black/ grey market drugs. These frameworks should be driven and overseen by global organizations (such as the WHO) to avoid the need for individual pharma companies to align with each nation state.
- New risk-sharing models to protect companies from the inevitable challenges that will arise when building new manufacturing capability and supply chains and transferring tech and IP, while ensuring the highest quality levels are maintained. This will lower risks and costs over time as capabilities scale.
- A shift in how technologically advanced companies share knowledge and train local populations to do advanced technical work. This requires collaboration with governments, universities and NGOs to build talent pools and put safeguards in place to protect investment in training and development. Ultimately this will create a larger, more diverse global talent pool.
- Innovating and operating in new countries and markets will require governments and policymakers to invest in local education and regulations to stimulate the right ecosystem capabilities to drive local R&D, manufacturing and business model innovation. Companies, in turn, can leverage this as they seek to develop new approaches to drug development, market access and distribution in developed markets.
Ultimately, rebalancing global supply chains and bringing innovative medicines and advanced manufacturing capabilities to developing regions will transform global healthcare in five key ways:
-
Improving local and global health
-
Improving the economies of developing nations
-
Improving the trust of citizens in their governments and ability to deliver quality healthcare
-
De-risking the pharmaceutical supply chain and therefore access around the world, providing better resilience globally
-
Opening new opportunities through access to a wider, more diverse talent pool, innovative manufacturing and distribution approaches, and the ability to effectively target new markets and patient populations



