
DOWNLOAD
DATE
Contact
One of the many consequences of the COVID-19 crisis has been difficulty in continuing to enroll and run clinical trials, which typically involve large numbers of people interacting in multiple geographies. Arthur D. Little (ADL) has pioneered and deployed a new, risk-based predictive analytical approach, powered by machine-learning technology. It enables pharma companies to make much better predictions and forecasts to support decision-making around key issues, such as adapting patient targets to favor less affected and recovering geographies, program extensions, and new trials. This approach is not just applicable to the current crisis – it is also a platform for next-generation transformation of clinical operations management.
The current challenges of managing clinical trials
Managing clinical trial operations effectively is critical for pharma companies. With typical costs of $20–50 million per trial, and multiple trials in operation at any one time, involving thousands of patients and sites across the world, operational planning and decision-making are complex. Delays, failures or operational gaps can destroy business value, and more importantly, increase the risk of patient harm if treatments are interrupted.
When the world is gripped by a crisis such as COVID-19, continuing to run large clinical trials is difficult, and some pharma companies have already responded by pausing some or all of their trial programs. There are several key challenges:
- Difficulty with patient recruitment if patients are homebound or reluctant to go to clinics.
- Clinics allowing only essential or critical visits or refusing to take part in trials.
- Recruited patients dropping out of trials.
- Staff or patients being placed at greater risk by exposure to the pandemic during trial operations.
- Vendors and contractors unable to meet obligations, such as delivering drugs to certain sites or continuing with site monitoring.
- Risks of compromised data integrity if arrangements deviate from the plan, e.g., having to move to home measurements or shortfalls in monitoring.
Not all pharma companies are in a position to pause their trial programs. There may be both ethical and commercial risks which mean that minimizing trial disruption becomes critical. Patient well-being commitments, market-access timelines, and corporate goals may all make pausing a trial difficult. Clinical operation leads therefore need to constantly review COVID-19 status, trends and predictions on factors such as infection rates and policy changes at trial sites, taking advice from epidemiologists and other specialists to make these risk-based decisions. Decisions confronting trials may include changing country footprints and sites, program extensions, new-trial virtualization, home-based infusion, site access, and changes to planned database lock, monitoring and screening activities. Because of the lead times and costs involved in making changes, having a robust baseline paired with a reliable forward perspective is critical.
However, the way in which COVID-19 progresses in specific geographies is still complex and hard to predict, so simple extrapolation from past and current data is insufficient.
How the COVID-19 Prediction Dashboard can help
ADL has developed and applied a powerful approach for collecting and integrating internal and external data, aggregating it into customized dashboards, and setting up a framework to support relevant clinical operations decisions – the COVID-19 Prediction Dashboard.
The new approach is powered by leading-edge machinelearning (ML) technology to enable greatly enhanced prediction and forecasting. This means the tool can integrate data from hundreds of data sources, combine it with company internal data, and provide context-specific customized forecasts and predictions to support decision-making. Predictions are refreshed continuously on the basis of new data, which is essential in a situation in which changes are rapid and knowledge is still evolving week by week, or even faster. The model is designed for use in situations in which data is uncertain or incomplete. In geographies where detailed data is not available, the model can draw inference from similar areas with better data coverage.
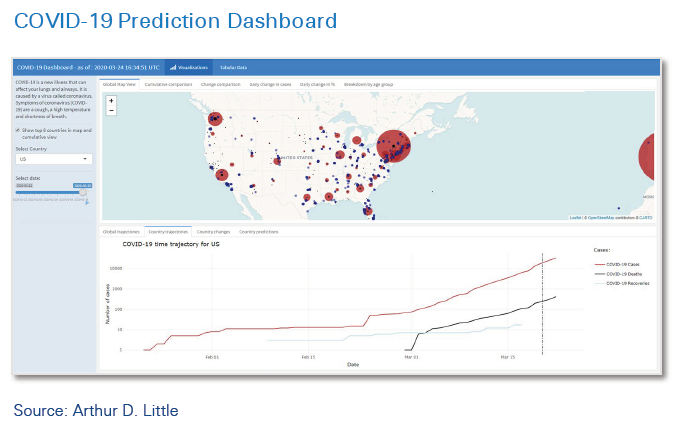
The COVID-19 Dashboard has a number of key features that make it transformative for decision-making, not just during the crisis, but also afterwards, as a key element of clinical operations management:
- Able to ingest both hard data from government, authorities, intelligence agencies and the like (there are currently about 160 unique sources), as well as “soft” data from sources such as Google searches and interaction, mobility, and demographic data.
- Combines external data with companies’ internal business and operational data to enable customized, context-specific analysis, aggregation and interpretation.
- Provides understanding of key risk indicators (KRIs) that need to be monitored for COVID-19 scenarios, ensuring that the most relevant data is monitored and reported.
- Integrates knowledge from companies’ in-house epidemiologists and other experts.
- Incorporates a machine learning-driven forecasting model with multiple prediction algorithms to understand the impact on relevant country/location disease trajectories.
- Allows what-if simulation modeling to assess scenarios (e.g., the impact of changes on country COVID-19 response policies).
- Provides customized, user-driven dashboard graphics which can be tailored for different user personas, including top leadership and operational staff.
Benefits of the COVID-19 Prediction Dashboard
Implementing the COVID-19 Prediction Dashboard offers multiple benefits to operational managers and top leadership during the crisis:
- Enables greatly enhanced predictive and forecasting capability, complementing and strengthening existing inhouse capabilities.
- Allows managers to understand clearly through heat maps where variances are significant across operations, which may require interventions or remediation.
- Uses company risk/issue trackers to look into studies and across studies and programs for one-time critical events, as well as systematic issues that would benefit future designs and operations.
- Improves the ability to anticipate and take action through identifying weak signals and early risk indicators of key trends.
- Provides a centralized data source, validated by individual functions, which clarifies linkages and knock-on effects and enables reallocation of clinical supplies from one site to another.
- Allows managers to prioritize activities and remediations once they can re-enter sites, e.g., where to focus on issues that are critical to patient safety or success of trials (such as data and audits).
- Enables better linkage between operational- and strategiclevel decision-making, using consistent data.
- Helps management teams interpret large and complex data sets more quickly and effectively for management decision-making.
- Enables data analysis on multiple levels: within a study or program, across programs, or across vendors (e.g., for upleveling to best practice).
- Ingests rolling data from active trials, and therefore has prior historic data from pre-event, event onset, and event progression, which allows managers to look for trends in studies.
- Provides a facilitated and documented approach, which can be kept in the trial master file to ensure consistency, transparency and compliance with ongoing guidance from authorities.
Implications for next-generation clinical operations
The Prediction Dashboard approach is much more than just a tool for COVID-19. It forms the foundation for nextgeneration transformation of clinical operations through a more dynamic and proactive approach to decision support and risk management – nearly all the benefits listed above are also invaluable for trial management under “normal” circumstances. Having a superior geographical intelligence and forecasting capability can help the business to:
- Gain first-mover advantage in selecting sites for trials.
- Achieve greater effectiveness and efficiency through access to forward-looking data on which to plan – rather than relying on historical data that may be weeks or months old.
- Stress-test trial plans using simulations to anticipate risks and improve resilience.
We are confident that this approach will be an essential platform for best-practice clinical operations going forward.
Applying the COVID-19 Prediction Dashboard
The initial COVID-19 Prediction Dashboard can be set up in as little as three weeks:
- In week 1 we work with your teams to create an initial dashboard sufficient for current and historical trial operations data based on your existing reporting frameworks. We examine key measures such as screening missed visits and drop-outs mapped against disease incidence; this also includes undertaking a detailed cause-and-effect analysis to understand COVID-19-centric impact.
- In weeks 2 and 3 we develop an ML-driven forecasting model to understand the impact of disease trajectories in relevant geographies and create customized forecasts in the context of trial objectives and programs.
In subsequent weeks we work with you to incorporate correlated risks and new data sets, update and develop forecast insight and strategies at country/site/study level, and continuously improve forecast algorithms to incorporate the latest thinking on disease modeling.
We do not provide just the dashboard itself, but an integrated support service provided by our leading consulting experts in clinical trial operations, risk management and business continuity, and digital solution development, including worldclass AI and ML expertise. The service is innovative and further development is ongoing, but we already have proven experience in client applications (see the case study opposite).
Case study – Applying the COVID-19 Prediction Dashboard at a mid-sized pharma company
ADL was engaged by a mid-sized pharma company to rapidly develop an integrated, global approach to understanding the impact of COVID-19 on all active and planned clinical trials, including patients, vendors and contractors, and relevant data and activities.
Working with the company’s in-house team, we developed an approach to collecting and integrating data (internal and external), aggregating and correlating it into focused dashboards, and setting up a framework to make or recommend relevant clinical operations decisions.
After setting up the initial framework and dashboard within three weeks, we have supported the company’s operational and leadership teams in making decisions on issues such as home-based infusion, site access, database lock, monitoring and screening.
The Prediction Dashboard model is constantly updated and refreshed as the work continues. The company has found the dashboard approach to be highly effective and plans to integrate the approach as a continuous platform for clinical trial management.
ADL’s COVID-19 Prediction Dashboard approach can greatly enhance the ability of pharma companies to manage clinical trials effectively, not just during a pandemic. The dashboard approach helps companies to:
- Get back control of clinical trial operations, rather than constantly chasing events.
- Look ahead to make better and more informed operational and strategic decisions.
- Gain competitive advantage through enhanced intelligence on trends affecting potential trial sites.
- Develop a framework and firm basis for planning and decision-making in the future.
- Manage risks better and improve resilience through adopting a forward-looking, dynamic approach.
Effective prediction capability will be essential for companies in the “new normal” after the crisis – not just in pharma, but across all industries.
If you would like to hear more about the COVID-19 Prediction Dashboard, please contact the ADL office near you.








